To understand ''reverse osmosis'', it is probably best to start with normal ''osmosis'' first.
According to Merriam-Webster's Collegiate Dictionary, Osmosis is the ''movement of a solvent through a
semipermeable membrane (as of a living cell) into a solution of higher solute concentration that tends to
equalize the concentrations of solute on the two sides of the membrane''. That's a mouthful. To understand
what it means, this picture is helpful: 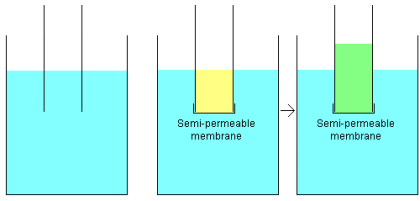
On the left is a beaker filled with water, and a tube has been half-submerged in the water. As you would expect, the water level in the tube is the same as the water level in the beaker. in the middle figure, the end of the tube has been sealed with a "semi-permeable membrane" and the tube has been half-filled with a salty solution and submerged. Initially the level of the salt solution and the water are equal, but over time something unexpected happens - the water in the tube actually rises. The rise is attributed to "osmotic pressure". A semi-permeable membrane is a membrane that will pass some atoms or molecules but not others. Saran wrap is a membrane, but it is impermeable to most everything we commonly throw at it. The best common example of a semipermeable membrane would be the lining of your intestines or a cell wall. Gore-Tex is another common semipermeable membrane. Gore-Tex fabric contains an extremely thin plastic film into which billions of small pores have been cut. The pores are big enough to let water vapor through, but small enough to prevent liquid water from passing. In the figure above, the membrane is able to allow passage of water molecules but not salt molecules. One way to understand osmotic pressure would be to think of the water molecules on both sides of the membrane. They are in constant brownian motion. On the salty side, some of the pores get plugged with salt atoms, but on the pure water side that does not happen. Therefore more water passes from the pure water side to the salty side as there are more pores on the pure water side for the water molecules to pass through. The water on the salty side rises until either: a) the salt concentration becomes the same on both sides of the membrane (which isn't going to happen in this case since there is pure water on one side and salty on the other), or b) the water pressure rises, as the height of the column of salty water rises, until it is equal to the osmotic pressure. At that point osmosis will stop. Osmosis, by the way, is why drinking salty water (for example ocean water) will kill you. When you put salty water in your stomach, osmotic pressure begins drawing water out of your body to try to dilute the salt in your stomach. Eventually you dehydrate and die. (sorry for the expression...) In reverse osmosis, the idea is to use the membrane to act like an extremely fine filter to create drinkable water from salty (or otherwise contaminated) water. The salty water is put on one side of the membrane and pressure is applied to stop, and then reverse, the osmotic process. It generally takes a lot of pressure, is fairly slow and wastes loads of water, but, it works! |
|
Click here to visit our Home Page
Click Here for our Reverse Osmosis Water Filters Lineup Page
Click Here for our Culligan Water Filter Products
Click Here for our Water Factory RO Filter Products
Click Here for most Replacement Cartridges
