HOME PAGE Customer Service
HOME PAGE Customer Service
WATER SOFTENERSThey make hard water easy to get along with.An Overview By: THOMAS KLENCK, PM
- The Fix
|
 |
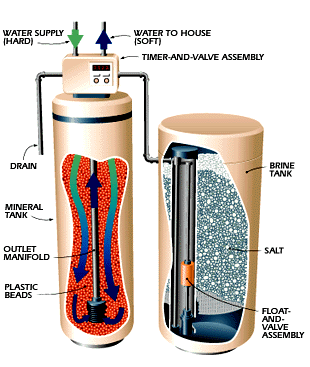
| 1. The backwash phase removes dirt from the mineral tank. 2. Recharging the mineral tank with sodium from the brine solution displaces calcium and magnesium, which is then washed down the drain. 3. The final phase rinses the mineral tank with fresh water and loads the brine tank so it's ready for the next cycle. |
The Fix
The solution to the problem is to get rid of the calcium and magnesium. While
there are chemical treatments that do this, the most popular answer is a water
softener.
The typical water softener is a mechanical appliance
that's plumbed into your home's water supply system. All water softeners use the
same operating principle: They trade the minerals for something else, in most
cases sodium. The process is called ion exchange.
The heart of a water softener is a mineral tank. It's
filled with small polystyrene beads, also known as resin or zeolite. The beads
carry a negative charge.
Calcium and magnesium in water both carry positive
charges. This means that these minerals will cling to the beads as the hard
water passes through the mineral tank. Sodium ions also have positive charges,
albeit not as strong as the charge on the calcium and magnesium. When a very
strong brine solution is flushed through a tank that has beads already saturated
with calcium and magnesium, the sheer volume of the sodium ions is enough to
drive the calcium and magnesium ions off the beads. Water softeners have a
separate brine tank that uses common salt to create this brine solution.
In normal operation, hard water moves into the mineral
tank and the calcium and magnesium ions move to the beads, replacing sodium
ions. The sodium ions go into the water. Once the beads are saturated with
calcium and magnesium, the unit enters a 3-phase regenerating cycle. First, the
backwash phase reverses water flow to flush dirt out of the tank. In the
recharge phase, the concentrated sodium-rich salt solution is carried from the
brine tank through the mineral tank. The sodium collects on the beads, replacing
the calcium and magnesium, which go down the drain. Once this phase is over, the
mineral tank is flushed of excess brine and the brine tank is refilled.
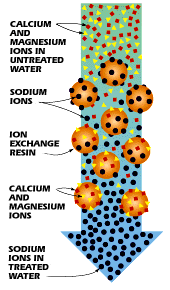 |
| In ion exchange, hard water ions replace sodium ions on beads. Process is reversed to flush minerals away. |
The Brains
Most popular water softeners have an automatic regenerating system. The most basic type has an electric timer that flushes and recharges the system on a regular schedule. During recharging, soft water is not available.A second type of control uses a computer that watches how much water is used. When enough water has passed through the mineral tank to have depleted the beads of sodium, the computer triggers regeneration. These softeners often have reserve resin capacity, so that some soft water will be available during recharging.
A third type of control uses a mechanical water meter to measure water usage and initiate recharging. The advantage of this system is that no electrical components are required and the mineral tank is only recharged when necessary. When it is equipped with two mineral tanks, softened water is always available, even when the unit is recharging.
Judging Water Hardness
Companies that sell water softening equipment generally offer test kits that help you determine the hardness of your water. For commercial testing sources, check your Yellow Pages under "water analysis."Water hardness is measured in grains per gallon (GPG) or milligrams per liter (mg/l, equivalent to parts per million, or ppm). Water up to 1 GPG (or 17.1 mg/l) is considered soft, and water from 1 to 3.5 GPG is considered moderately hard. Water from 3.5 to 7 GPG is Hard Water, and from 7 to 10.5 GPG is Very Hard. A water softener's effectiveness depends on how hard the incoming water is. Water over 100 GPG may not be completely softened.
Health Concerns
Hard water poses no health hazard. On the other hand, the sodium that remains in softened water may be a problem for those on sodium-restricted diets. Other people simply may wish to avoid the slightly salty taste of treated water. In either case you can install a separate water dispenser that bypasses the softener. You also can use potassium chloride instead of salt, although this costs about three to four times more.Click here to visit our Home Page
Click Here for our Water Softeners Lineup Page
Click Here for most Replacement Cartridges